UNDERSTANDING MASTER FILES
Master Files (MFs) are reference documents that contain proprietary and/or publicly-available data on aspects of drug or Natural Health Product (NHP) safety, efficacy, and/or quality that are submitted to regulatory authorities in support of various application types, including Clinical Trial Applications (CTAs), Investigational New Drugs (INDs), Abbreviated New Drug Applications or Submissions (ANDAs / ANDS), New Drug Applications or Submissions (NDAs / NDS), and Product Licence Applications (PLAs).
MASTER FILES SUBMISSION
MFs are submitted by individuals or companies that develop, manufacture, or supply drugs or NHPs, and are known as the MF Holders. In some jurisdictions the entire MF is strictly confidential between the MF Holder and the regulatory Agency, whereas in others restricted access is granted to Applicants authorized by the MF Holder. Once authorized, the Applicant is allowed to reference the MF in their own submission(s).
The purpose of a MF is to provide sufficient details to the regulatory Agency to satisfy safety, efficacy, and/or quality requirements. The information used in a MF can include specifics about the facilities, processes, and components used in manufacturing, processing, packaging, and storage, without disclosing proprietary information to the Applicant. The MF Holder is responsible for maintaining the MF and ensuring that it is kept up-to-date throughout its lifecycle.
TYPES OF MASTER FILES BY JURISDICTION
In Canada, an NHP-MF serves the same purpose and follows a similar submission process as that of a Drug Master File (DMF). The United States (US) also has DMFs, whereas they are referred to as Active Substance Master Files (ASMFs) in Europe – both regions do not have a MF pathway for dietary or food supplements.
The type of MF needed depends on the regulated category and information intended to be communicated to the regulatory authority. For example, Health Canada and the US Food and Drug Administration (FDA) recognize five (5) different types of DMFs, yet the European Medicines Agency (EMA) only uses one (i.e., ASMF). Health Canada’s Natural and Non-prescription Health Products Directorate (NNHPD) also has a standalone MF specific to NHPs. The table below provides a summary of the different MF types across each of the three jurisdictions.
FIND OUT MORE:
Nutrasource’s NHP & Pharmaceutical Regulatory Sciences team is well-versed in MF dossier preparation and submission to various regulatory authorities.
Our experts can help navigate between MF types and the requirements for each section. Contact us today.
REFERENCES:
4. Drug MF https://www.fda.gov/drugs/guidances-drugs/drug-master-files-guidelines
5. Drug MF https://www.fda.gov/drugs/forms-submission-requirements/drug-master-files-dmfs
6. US Dietary Supplement https://www.fda.gov/news-events/press-announcements/fda-updates-draft-guidance-premarket-safety-notifications-dietary-supplement-industry
7. Drug MF https://www.fda.gov/media/85079/download
8. Active Substance Master File https://www.ema.europa.eu/en/documents/report/final-guideline-active-substance-master-file-procedure-revision-4_en.pdf




.jpg?width=1792&height=1344&name=Drug%20Submission%20Flowchart%20(6).jpg)
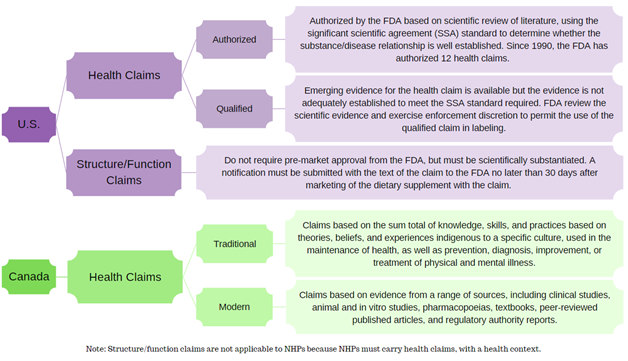
.png?width=1080&name=Claims%20dos%20and%20donts%20(1).png)
-1.png?width=711&height=197&name=Related%20Content%20-%20for%20website%20pages%20(1)-1.png)