You've developed a new product and are keen to market it as a dietary supplement in the U.S. You're confident it fulfills the FDA's definition, but its "star" ingredient is newer to the market and, to your knowledge, hasn't been used in foods to date. This means that if you marketed it as is, the product would be considered adulterated.
What options do you have to go to market, and what is the most effective way to do so without raising regulatory red flags and risking your brand reputation?

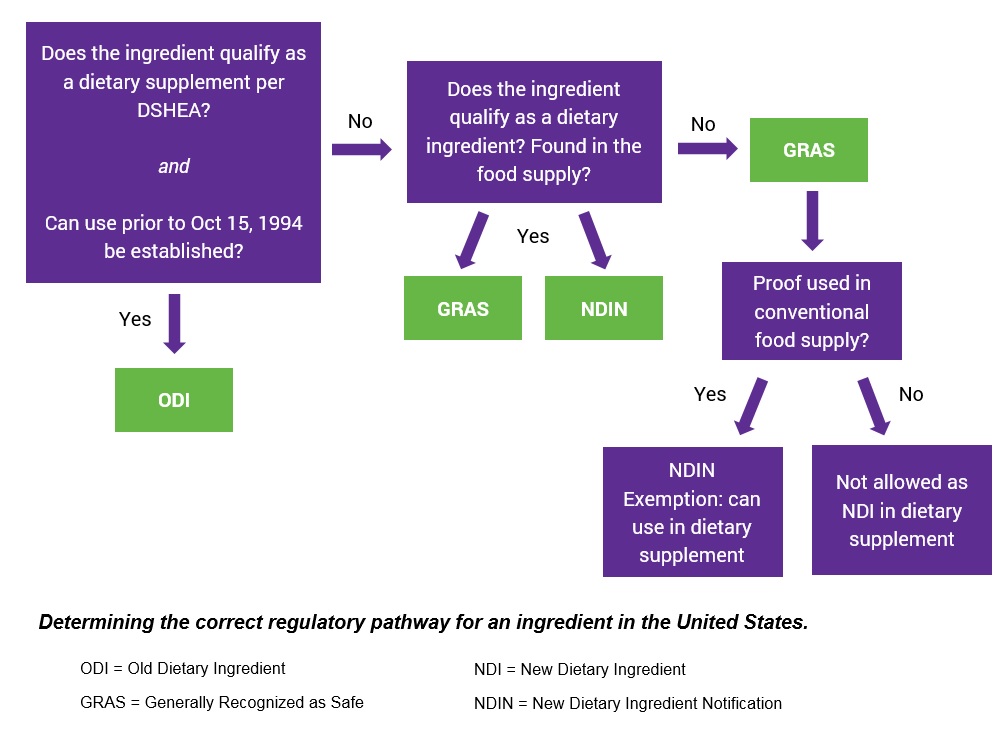
What to Consider When Choosing GRAS or NDIN
There are two different regulatory routes that make sense in this case: NDIN (New Dietary Ingredient Notification) or GRAS (Generally Recognized as Safe).
Both NDIN and GRAS have skyrocketed in popularity in recent years because of how effectively they allow companies to launch innovative ingredients safely, compliantly, and with minimal risk once approved.
Whether you choose NDIN or GRAS depends on a number of factors, including:
- Whether the ingredient qualifies as a dietary supplement per DSHEA (Dietary Supplement Health and Education Act of 1994)
- If use can be established prior to October 15, 1994
- Whether the substance is found in the food supply
- The level of safety evidence that exists
- How "proprietary" you want your formulation, and its supporting data, to be
- Which populations you wish to target and whether the product has safety concerns for specific groups (e.g., pregnant and lactating women, children under 3 years of age, etc.)
- The product's organoleptic properties (e.g., smells or tastes not desirable in foods)
- Your desired timeline from submission to FDA to approval
- Considerations for certain ingredients (e.g., probiotics, nature-identicals, etc.)
The Main Differences Between GRAS and NDIN
To help you determine which regulatory route is right for your ingredient, below is a brief side-by-side comparison of GRAS vs. NDIN:
NDIN |
GRAS |
|
|
Where to Go From Here
Overall, determining the correct path for your ingredient can be tricky, but ultimately it is a necessary step to maximizing the market success of your product.
Working with an experienced consultant with longstanding expertise in GRAS and NDIN filings, like SGS Nutrasource and GRAS Associates, will expedite the process and ensure you have all the information you need to successfully gain FDA approval.
With our 97% success rate, we will help you navigate the regulations, submit your NDIN or GRAS filing, and liaise with the FDA on your behalf. Contact our expert regulatory team to get started today.
 Amy Mozingo, MS, is Vice President of US Nutra Regulatory Sciences. She has over 15 years of experience in industry and consulting, holds a certificate as a Preventive Control Qualified Individual (PCQI), and is trained and experienced in ingredient approvals (GRAS, NDIN, FAP, CAP), product labelling, formulation reviews, and current good manufacturing requirements for dietary supplements. Connect with Amy on LinkedIn.
Amy Mozingo, MS, is Vice President of US Nutra Regulatory Sciences. She has over 15 years of experience in industry and consulting, holds a certificate as a Preventive Control Qualified Individual (PCQI), and is trained and experienced in ingredient approvals (GRAS, NDIN, FAP, CAP), product labelling, formulation reviews, and current good manufacturing requirements for dietary supplements. Connect with Amy on LinkedIn.

