Thinking about getting your toxicological results published? Go no further until you read this article.
Posted by Nutrasource on Tue, Oct 22, 2024
Thinking about getting your toxicological results published? Go no further until you read this article.
Tags: Claims, Regulatory, Concept to Claim, market access
Thinking about placing a toxicological test on your novel ingredient? Go no further until you read this article.
Tags: Claims, Regulatory, Concept to Claim, market access
Posted by Nutrasource on Thu, Jul 25, 2024
You may have a blockbuster food or dietary supplement ingredient, but if the manufacturing process introduces contaminants, it may not be safe to consume. Contaminants may be introduced into a botanical ingredient during growth, such as heavy metals or herbicides from soil or pesticides or antifungal agents applied to plants. Toxins can be produced by plants or be introduced into plants during storage by fungal contamination. Ingredients that are produced by chemical synthesis may be contaminated with solvents or unintended metabolites, and ingredients that are manufactured by microbial fermentation can be contaminated with the microbes or toxins produced by them. Other sources of contaminants include water, processing aids, filters/columns, fermentation media ingredients and packaging materials. Use of Good Manufacturing Practice (GMP), potable water and food grade materials (by U.S. standards) can help reduce levels of contaminants in an ingredient. However, even if these procedures are in place, contaminants may be present in your ingredient.
Tags: Claims, Regulatory, Concept to Claim, market access, Animal Supplements
Posted by Nutrasource on Fri, Aug 11, 2023
Tags: Claims, Regulatory, Concept to Claim, market access, Animal Supplements
Posted by Nutrasource on Tue, Jun 06, 2023
-1.png?width=657&height=197&name=Certifications%20newsletter%20%20(5)-1.png)
The dietary supplement industry is growing rapidly in South Korea. On a case by case basis, Korean-manufactured products can now showcase a third-party certification label on their product packaging – the International Fish Oil Standards (IFOS™) certification label. This is a way for brands to distinguish themselves in a highly competitive market while building consumer trust.
Continue reading to learn how Certifications by Nutrasource can assist with achieving this certification.
.png?width=718&height=539&name=IFOS%20process%20in%20Korea%20(1).png)
Once the application has been approved, the IFOS™ logo can be added to product packaging and advertisements for Korean-manufactured Omega-3 supplements. This signifies that the product is reviewed and approved by the KHSA, providing consumers with added assurance of safety and quality.

Nutrasource Asia can assist Korean supplement brands in obtaining IFOS™ certification and reaping its benefits. Here's how:
Introduction to the IFOS™ program:
Nutrasource Asia provides expertise to Korean brands on obtaining IFOS™ certification. Despite the high awareness of IFOS™ in the Korean market, many supplement brands are not familiar with the program's origin or the certification process. The team can guide you through the entire process of obtaining IFOS™ certification.
English Language support:Nutrasource Asia offers a professional solution for brands who require assistance in communicating with the Certifications by Nutrasource team in Canada but do not speak English. The team at Nutrasource Asia can seamlessly manage the entire process, from contracting to sending samples to Canada to receiving IFOS™ certification, ensuring a smooth and hassle-free experience for clients.
Assistance with market access strategy:
Nutrasource Asia offers a range of services to help clients navigate the complex regulatory landscape in Korea. Our team can provide guidance on Korean regulations, assist with the IFOS™ certification process, and explain the product launch process in Korea. Additionally, we offer support in coordinating production and testing plans to ensure a seamless market launch for your products.
Market monitoring for IFOS™ misuse or abuse:
Nutrasource Asia provides ongoing monitoring of the Korean marketplace for clients who have obtained IFOS™ certification, ensuring that the certification is not misused or abused. If any issues are identified, Nutrasource Asia works with clients to promptly correct them.
By following this step-by-step guide and working with Nutrasource Asia, eligible Korean brands can obtain an IFOS™ certification and take advantage of the program's benefits.
Ultimately, IFOS™ helps brands differentiate themselves in a competitive market and meet the demands of quality-conscious consumers.
Learn more the IFOS™ program.
RELATED CONTENT:
Tags: Product Testing & Certifications, Omega-3s, Dietary Supplements/Natural Health Products, market access
Posted by Kevin Yan, M.Sc., VP Certifications & Analytics on Tue, Apr 25, 2023
Tags: Product Testing & Certifications, Omega-3s, Probiotics, Dietary Supplements/Natural Health Products, Non-GMO, market access
Posted by Santa Al Antwan, Regulatory Affairs Associate on Wed, Feb 22, 2023
As a result of the pandemic, respiratory and seasonal illnesses, and other emerging threats, consumers are more interested, invested, and involved in their wellbeing than ever before. When it comes to health products, consumers are motivated by those that are clean or naturally-sourced, convenient, scientifically substantiated, and tailored to meet their personal needs. Thus, driving industry trends towards transparency, sustainability, personalization, and virtual approaches.
Tags: Product Testing & Certifications, Product Marketing, Regulatory, Dietary Supplements/Natural Health Products, market access
Posted by Paula Guerra. Regulatory Affairs Manager on Tue, Jan 24, 2023
Companies looking to obtain authorization to market a new pharmaceutical must either file a New Drug Submission (NDS) to Health Canada or a New Drug Application (NDA) to the United States Food and Drug Administration (U.S. FDA), depending on their market-of-interest.
The regulatory dossier must include data around the following:
In summary, what this means is that the regulatory dossier must provide enough information to ensure the safety, efficacy, and quality of the drug product, showing that the benefits of the drug outweigh the risks.
Common Technical Document (CTD)
The information to be presented to the Agency is organized into five (5) modules, using the CTD format, as follows:
Process and Timeline
.jpg?width=1792&height=1344&name=Drug%20Submission%20Flowchart%20(6).jpg)
As seen in this flowchart, the entire regulatory journey from the initial drug application to its approval takes approximately 6 months for drugs that qualify for accelerated approval and up to 2 years for drugs that follow regular processing timelines, avoiding any delays.
Here are some key points of what happens at each stage:
SGS Nutrasource offers full-service consulting in this to make sure the new drug submission process is smooth. The team of regulatory experts extensively researches, compiles, and reviews drug submissions for compliance in Canada and/or the U.S. and files the application to the appropriate Agency, helping clients to achieve regulatory confidence and market access.
Speak to a member of our team on how we help you successfully bring your pharmaceutical to market.
RELATED CONTENT
Tags: Regulatory, Dietary Supplements/Natural Health Products, market access
Posted by Santa Al Antwan, Regulatory Affairs Associate on Wed, Nov 23, 2022
In today’s regulatory climate of full accountability and transparency, it is critical to have strong science to back-up any claim made on a health product, be it a dietary supplement sold in the United States (U.S.) or a Natural Health Product (NHP) marketed in Canada. This will be a key component of your dietary supplement and NHP marketing strategy. We know advertising health products is critical to your marketplace success and we want to help you make sure it is done the right way.
Firstly, it is important to note that while dietary supplements are equivalent to the NHP categories, there are some major differences in regulations between the two countries.
| U.S. | Canada |
|
|
|
|
As a result, the potential claims for dietary supplements and NHPs vary significantly with most dietary supplements carrying structure/function claims and NHPs ranging from low- to high-risk health claims.
Given the complexity and nuances between jurisdictions, SGS Nutrasource’s regulatory experts will help you understand how to approach claims substantiation and apply these best practices to both product types.
Label Claims for Dietary Supplements – in US and Canada
In the U.S., dietary supplements may make health claims; however, they are subject to pre-market review and authorization by the U.S. FDA. Structure/function claims are not pre-approved per se, but a notification must be submitted to the FDA no later than 30 days after the dietary supplement is first marketed.
In Canada, an NHP must have a recommended use expressed via its health claim, and that health claim must have a health context in order to attain Health Canada approval in the form of a Natural Product Number (NPN). Therefore, given the conditions under which NHPs are screened, reviewed, and subsequently approved, they cannot carry structure/function claims.
This flowchart summarizes the different categories of claims in the U.S. and Canada.
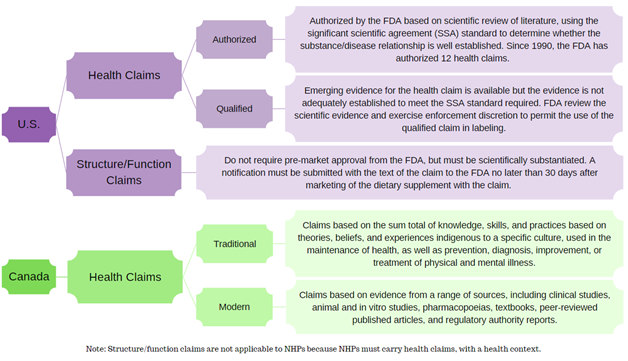
Constructed using guidance documents from the FDA and Health Canada.
Simply put, in order to avoid unnecessary and potentially costly consequences, we need to know the do’s and don’ts to consider when assessing a product’s claims potential.
.png?width=1080&name=Claims%20dos%20and%20donts%20(1).png)
SGS Nutrasource offers claims substantiation consulting to ensure regulatory compliance for your product label in line with the various geographical jurisdictions.
Speak to a member of our team on how we can support your regulatory needs.
RELATED CONTENT
Tags: Claims, Regulatory, Dietary Supplements/Natural Health Products, market access
Posted by Nutrasource on Wed, Jun 29, 2022
Summary: FDA announces draft guidance for industry on their policy to exercise enforcement discretion on delinquent notifications to encourage firms to submit NDIN to correct past failures. Read on to learn more.
Tags: Product Testing & Certifications, Regulatory, Concept to Claim, ingredients, market access