Thinking about getting your toxicological results published? Go no further until you read this article.
Posted by Nutrasource on Tue, Oct 22, 2024
Thinking about getting your toxicological results published? Go no further until you read this article.
Tags: Claims, Regulatory, Concept to Claim, market access
Thinking about placing a toxicological test on your novel ingredient? Go no further until you read this article.
Tags: Claims, Regulatory, Concept to Claim, market access
Posted by Nutrasource on Thu, Jul 25, 2024
You may have a blockbuster food or dietary supplement ingredient, but if the manufacturing process introduces contaminants, it may not be safe to consume. Contaminants may be introduced into a botanical ingredient during growth, such as heavy metals or herbicides from soil or pesticides or antifungal agents applied to plants. Toxins can be produced by plants or be introduced into plants during storage by fungal contamination. Ingredients that are produced by chemical synthesis may be contaminated with solvents or unintended metabolites, and ingredients that are manufactured by microbial fermentation can be contaminated with the microbes or toxins produced by them. Other sources of contaminants include water, processing aids, filters/columns, fermentation media ingredients and packaging materials. Use of Good Manufacturing Practice (GMP), potable water and food grade materials (by U.S. standards) can help reduce levels of contaminants in an ingredient. However, even if these procedures are in place, contaminants may be present in your ingredient.
Tags: Claims, Regulatory, Concept to Claim, market access, Animal Supplements
Posted by Nutrasource on Fri, Aug 11, 2023
Tags: Claims, Regulatory, Concept to Claim, market access, Animal Supplements
Posted by Santa Al Antwan, Regulatory Affairs Associate on Wed, Nov 23, 2022
In today’s regulatory climate of full accountability and transparency, it is critical to have strong science to back-up any claim made on a health product, be it a dietary supplement sold in the United States (U.S.) or a Natural Health Product (NHP) marketed in Canada. This will be a key component of your dietary supplement and NHP marketing strategy. We know advertising health products is critical to your marketplace success and we want to help you make sure it is done the right way.
Firstly, it is important to note that while dietary supplements are equivalent to the NHP categories, there are some major differences in regulations between the two countries.
| U.S. | Canada |
|
|
|
|
As a result, the potential claims for dietary supplements and NHPs vary significantly with most dietary supplements carrying structure/function claims and NHPs ranging from low- to high-risk health claims.
Given the complexity and nuances between jurisdictions, SGS Nutrasource’s regulatory experts will help you understand how to approach claims substantiation and apply these best practices to both product types.
Label Claims for Dietary Supplements – in US and Canada
In the U.S., dietary supplements may make health claims; however, they are subject to pre-market review and authorization by the U.S. FDA. Structure/function claims are not pre-approved per se, but a notification must be submitted to the FDA no later than 30 days after the dietary supplement is first marketed.
In Canada, an NHP must have a recommended use expressed via its health claim, and that health claim must have a health context in order to attain Health Canada approval in the form of a Natural Product Number (NPN). Therefore, given the conditions under which NHPs are screened, reviewed, and subsequently approved, they cannot carry structure/function claims.
This flowchart summarizes the different categories of claims in the U.S. and Canada.
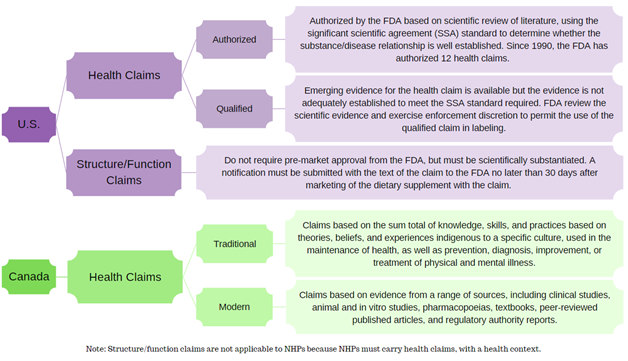
Constructed using guidance documents from the FDA and Health Canada.
Simply put, in order to avoid unnecessary and potentially costly consequences, we need to know the do’s and don’ts to consider when assessing a product’s claims potential.
.png?width=1080&name=Claims%20dos%20and%20donts%20(1).png)
SGS Nutrasource offers claims substantiation consulting to ensure regulatory compliance for your product label in line with the various geographical jurisdictions.
Speak to a member of our team on how we can support your regulatory needs.
RELATED CONTENT
Tags: Claims, Regulatory, Dietary Supplements/Natural Health Products, market access
Posted by Ruth Rodrigues, Marketing Manager on Thu, May 05, 2022
Increasingly, over the past few years, the dietary supplement industry has emphasized quality control and claim substantiation. At times, companies conserve on quality control and can end up with a product that doesn't match the claims and ingredients on the label. Many look to third-party organizations that help solve this problem by objectively certifying supplements for purity, safety, and quality. Non-GMO certification has become an useful tool to address some of these concerns. Keep reading to learn more about consumer demand for third-party tested products and ingredients and learn more about the Non-GMO certification process.
Consumers today are far more educated and discerning in their choice of dietary supplements. For context, one in five of US consumers make a transparent vitamin and supplement brand their first choice, according to a consumer market research study, as they seek transparency and third-party certification to back up their claims. Outlined in the 2021 TTC ITC Insights Consumer Supplement User survey, 31% of consumers consider quality certifications and seals on labels/ website a key driver of trust.
This goes beyond consumers, when healthcare practitioners were surveyed, over half of the dietitians surveyed considered quality certifications and seals displayed as the number one driver of trust.
Needless to say, having third-party certifications back your claims is critical both for consumer consumption and healthcare practitional referral of product. As mentioned, one area that leading dietary supplement brands have leveraged is third-party certifications related to Non-GMO testing. Not only are consumers looking for Non-GMO tested products and ingredients, many retailers require a brand to have third-party certification validating their Non-GMO claims.
Making a Non-GMO claim through traditional supply chain verification is impossible for some ingredients. Certifications by Nutrasource has a streamlined and effective process that assists your product and/or ingredient in acheiving these certifications.
Below we address some of the common questions we receive related to Non-GMO testing and certification.
Tags: Product Testing & Certifications, Product Marketing, Claims, Regulatory, Non-GMO
Posted by Ruth Rodrigues on Wed, Jan 12, 2022
| Budget, timelines, and pressure to be innovative are just a few of the barriers that many companies face when looking to gain market access. The trick is knowing what you want to say about your product, what you can say, and having a clear understanding of the marketplace you’re looking to penetrate. From there, a strategic solution can be developed to assist in achieving your goals. |
Tags: Product Marketing, Claims, Concept to Claim, Dietary Supplements/Natural Health Products
Posted by Nutrasource on Thu, Aug 20, 2020
Developing dietary supplements with strong claims not only allows brands to stand out from the pack but also provides a competitive advantage.
Tags: Claims, Regulatory, Concept to Claim, Dietary Supplements/Natural Health Products
Posted by Douglas Kalman, Ph.D., R.D., CCRC, FACN, Vice President of Scientific Affairs on Wed, May 06, 2020
Sports Nutrition as a business category within foods and dietary supplements have had a relatively short history. From a mass market perspective, it was not until 1965 and into the 1970’s that the nephrologist (kidney specialist) Robert Cade, MD started tinkering with a “homemade” beverage, later named Gatorade (Dr. Cade was the Director of the Renal Division at the University of Florida Medical School) for the sole purpose of helping football players stay hydrated during the hot and humid game conditions in Florida.
Tags: Clinical Trials, Claims, Regulatory, Sport Nutrition
Posted by Amy Mozingo, MS, Director of Operations - GRAS Associates on Wed, Apr 22, 2020
As we are all adjusting to modifying our work practices to stay safe and “flatten the curve” during the time of COVID-19, work must go on. R&D, product development, and regulatory compliance are as important as ever as the FDA has been handing out warning letters for unsubstantiated coronavirus claims.
Tags: Clinical Trials, Claims, Regulatory