Nutrasource Blog
GLP-1 Receptor Agonists: Reshaping Dietary Supplement Innovation
Posted by Dr. Stephanie-Anne Girard on Wed, Aug 13, 2025
Tags: Clinical Trials
Decentralized Clinical Trials: What you need to know about remote studies
Posted by Nutrasource on Thu, Mar 23, 2023
Content contributed by
.png?width=560&height=78&name=Article%20Contribution%20-%20hubspot%20size%20(9).png)
.png?width=560&height=78&name=Article%20Contribution%20-%20hubspot%20size%20(10).png)
Decentralized clinical trials (DCTs), also termed “virtual trials”, “site-less trials”, or “direct-to-participant trials”, are defined as the execution of clinical trials with the help of virtual visits or mobile healthcare providers, using technologies and methods that differ from a traditional clinical trial setup. DCTs can refer to both fully DCTs (virtual) and hybrid DCTs. In a fully decentralized clinical trial, participant recruitment, consenting, administration of study medication, collection of data, etc. will be done with no in-person study visits. In hybrid DCTs, most of the study procedures and visits are performed remotely, but the study participants may need to be on-site for some procedures such as physical examinations, sample collection, complex treatments, etc. The complexity of a clinical trial and the target population are key parameters that dictate whether to opt for a fully DCT, a hybrid DCT, or classic on-site trial approach to achieve the best results.
Virtual technologies
DCTs have also revolutionized the development and implementation of digital health technologies (DHTs) to support clinical research and collect real-time data remotely from the study participants. DCTs require various virtual tools and setups such as virtual visits, wearable medical devices, phone apps, etc., which are all examples of DHTs.

Are decentralized trials really the future of clinical trials?
There are some advantages of DCTs over traditional clinical trials. Conducting virtual visits can reduce the participant and caregiver burden (e.g., travel, parking, waiting time for an appointment). Wearable devices may also be utilized to monitor vital signs such as heart rate, respiration, blood pressure, and temperature remotely, which could help in early detection of treatment-related adverse effects. Such devices can also track activities of daily living, such as sleep duration and quality, step count, time in sedentary positions vs. walking/exercising, etc. DCTs can cover a broader geographical area and can target wider demographic groups. Using DHTs can help study participants and caregivers with study alerts and reminders, scheduling of study visits, and remote data collection. The US FDA has recently proposed that DCTs might reduce barriers and cater to a wider range of participants by reducing the costs and commitments required for study participants to take part in a clinical trial.
Potential limitations of DCTs
In on-site clinical trials, the study products are shipped to a clinical trial site and distributed to study participants by trained site staff. In DCTs, the study products are shipped directly to the study participants. It may be a challenge to monitor and maintain the proper temperature for study products during transit, which could jeopardize stability and thereby have a negative consequence on study data. Additionally, the protection of privacy and health information is another challenge in DCTs with the use of DHTs. The solicitation, collection, and secure storage of study participant health information requires a thorough understanding of various complex regulatory requirements that vary by region and systems. Identity verification, secure data access and training are critical to addressing this complexity. Study participants need thorough training on the use of technologies for data entry and data capture, including wearable devices and phone applications. The uninterrupted data collection from the DHT gadgets will require a proper cellular plan or wi-fi connection and availability of an IT support team to troubleshoot any technical issues and answer participants’ questions. At the center of technology is also the validity of the software and devices and compliance with federal regulations and international guidelines on clinical research data.
Misconceptions
There are some misconceptions in the dietary supplement industry that DCTs are less costly than traditional on-site trials. While there are benefits to the participant burden and potential decrease in recruitment timelines, there is a shift in labour required for the oversight and quality of the project which is focused more heavily on proper design, deployment and support of software and devices, and the costs for the software platform licensing and devices themselves, which offsets any time savings and costs a site may incur. Further, the costs associated with site staff remain nearly the same as the terms “virtual” and “site-less” are misleading. In these trials, there is still a requirement for site staff to support a DCT by interacting with the participants, performing follow-ups, managing medical oversight, being available for questions, etc. While there may be cost savings for studies such as observational trials, trials requiring thousands of participants, as well as for trials focused on rare indications/diseases, these cost savings are negligible in the dietary supplement industry where recruitment is less challenging due to the nature of the simpler study designs, smaller sample sizes, and more inclusive target populations.
Conclusion
The regulatory bodies need to work together to develop harmonized policies to support DCTs, ensure data integrity and protect sensitive health information of participants involved in the research. Virtual medicine and technologies have gained popularity over the past few years and are now being practiced and used at a larger scale. DCTs are becoming another tool in the clinical research industry alongside traditional clinical trials requiring brick-and-mortar sites, each with their own place and purpose. The successful execution of a decentralized clinical trial is tremendously dependent on the design, operational planning, and continuous oversight of the study. Collaborating with an experienced Contract Research Organization from trial conception is the key to ensuring that the study meets the highest standards of research.
CONTACT US
Exploring options for your clinical trial? Reach out to our team who would be glad to connect and help answer any questions you may have.
RELATED CONTENT
Tags: Clinical Trials, Pharmaceuticals
The State of Sport Nutrition: a peek into the multi-billion dollar industry
Posted by Douglas Kalman, Ph.D., R.D., CCRC, FACN, Vice President of Scientific Affairs on Wed, May 06, 2020
Sports Nutrition as a business category within foods and dietary supplements have had a relatively short history. From a mass market perspective, it was not until 1965 and into the 1970’s that the nephrologist (kidney specialist) Robert Cade, MD started tinkering with a “homemade” beverage, later named Gatorade (Dr. Cade was the Director of the Renal Division at the University of Florida Medical School) for the sole purpose of helping football players stay hydrated during the hot and humid game conditions in Florida.
Tags: Clinical Trials, Claims, Regulatory, Sport Nutrition
NDIN’s and Structure Function Claims in the Time of COVID-19
Posted by Amy Mozingo, MS, Director of Operations - GRAS Associates on Wed, Apr 22, 2020
As we are all adjusting to modifying our work practices to stay safe and “flatten the curve” during the time of COVID-19, work must go on. R&D, product development, and regulatory compliance are as important as ever as the FDA has been handing out warning letters for unsubstantiated coronavirus claims.
Tags: Clinical Trials, Claims, Regulatory
Clinical Trial Research Solutions During Social Isolation
Posted by Derek Tobin, Ph.D. on Tue, Apr 07, 2020
The current pandemic situation highlights a weakness in our traditional approach to clinical trials. You are probably experiencing some form of lock-down (“stay at home order” by your local or national government). Our innate sense of optimism says this crisis will blow over fast, but there are also dark clouds suggesting weeks may turn to months and true return to normality will take longer than we dare to think.
Tags: Clinical Trials, Concept to Claim, Foods & Beverages, Dietary Supplements/Natural Health Products
How to Validate Your Products Through Clinical Research
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Thu, Feb 13, 2020
Whether you are launching a new product or looking to substantiate a novel claim, strong science is crucial to the success of your health product. In Canada, many ingredients have been “pre-cleared” for specific claims at particular doses. Monographs give guidance and provide substantiation for these ingredients, so you don’t have to.
Tags: Clinical Trials, Dietary Supplements/Natural Health Products
Top 3 Reasons to Conduct Clinical Research in Canada
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Tue, Aug 20, 2019
Clinical research is an important investment for the entire supply chain, from ingredient companies to brands selling finished health products. Primary research serves many purposes, and is most commonly used to validate efficacy, safety, and substantiate claims that increase the impact of a company’s marketing messages.
Tags: Clinical Trials
How to Form a CRO Partnership That Delivers Results
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Mon, Oct 15, 2018
The term "partnership" has become a trendy word in the contract research organization (CRO) world. While it's meant to describe a close collaborative relationship between CRO and client, too many organizations claim to be partners when in reality they are only research sites or project-based consultants.
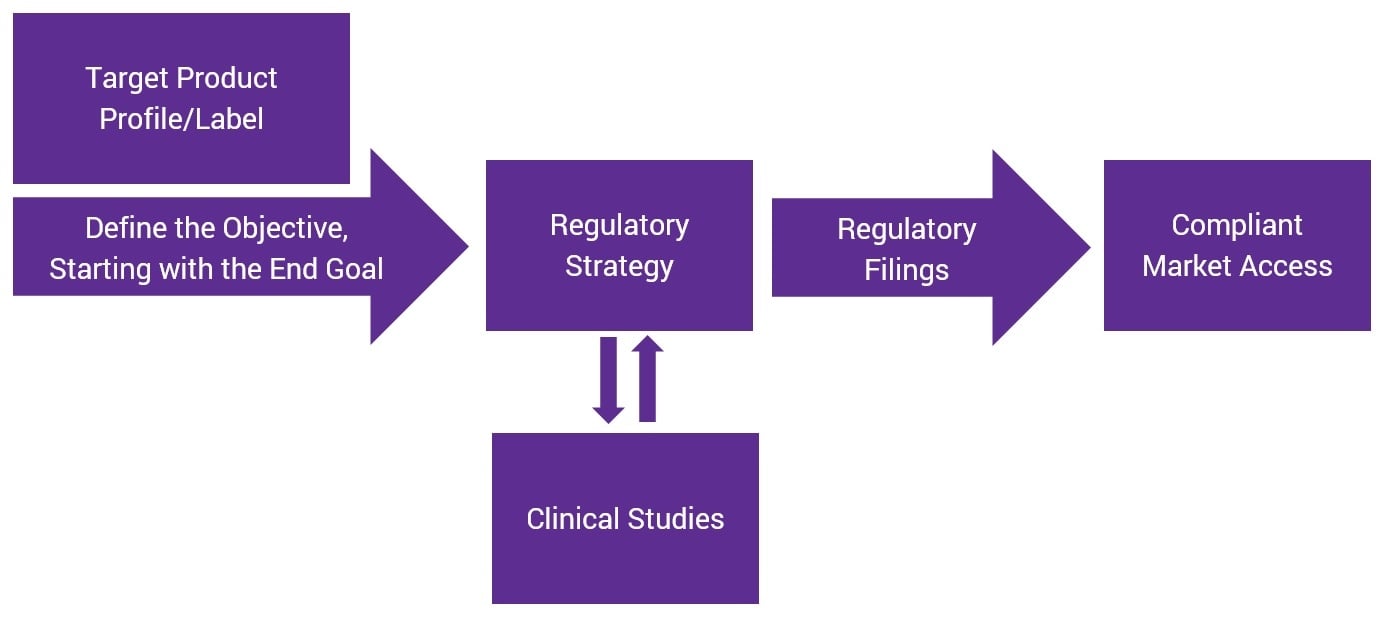
Reverse-Engineering Your Regulatory Strategy for More Efficient Product Development
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Thu, Jul 12, 2018
Regulatory strategy is the backbone of successful product development. Brands that lack a clear regulatory plan at the beginning of the process can face longer times to market and potentially higher costs associated with re-work or failure.
Tags: Clinical Trials, Regulatory, Dietary Supplements/Natural Health Products
What to Expect When Conducting Dietary Supplement Clinical Trials
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Tue, Mar 20, 2018
One of the most common questions we hear from clients early in the product lifecycle is: "How do I let my customers know our product is safe, and that it works?"
Tags: Clinical Trials


-2.png?width=657&height=183&name=Related%20Content%20-%20for%20website%20pages%20(1)-2.png)
-1.png)
.png)
.png)
-1.png)