While many U.S. states (and, of course, Canada) are approving medical and recreational use of cannabis products, confusion about how these products are regulated continues to grow.
Nutrasource Blog
Substantiating Products With No Guidance: The CBD Confusion
Posted by Susan J. Hewlings, Ph.D., R.D., Director of Scientific Affairs on Fri, Aug 17, 2018
Tags: Claims, Regulatory, Cannabis
Implications of FDA's GRAS and NDIN Policy Changes for Supplements and Medical Foods
Posted by Dr. Richard Kraska, Ph.D., Chief Scientific Officer - GRAS Associates on Tue, Jul 24, 2018
Our regulatory affairs team has regular contact with the U.S. Food and Drug Administration (FDA) for a number of projects. Occasionally we learn about subtle policy shifts regarding Generally Recognized as Safe (GRAS) Notifications and New Dietary Ingredient Notifications (NDIN).
Below is an overview of FDA’s latest updates to GRAS and NDINs which will have important implications for the dietary supplement and medical foods industry in the U.S.
Tags: Regulatory, Dietary Supplements/Natural Health Products
Next Steps for Commercializing Medical Cannabis Products in Canada, the U.S., and Beyond
Posted by Nutrasource on Tue, Jul 17, 2018
Canada has made history as the second country to legalize cannabis nationwide. The world is watching to see how the landscape for cannabis and related products will change as the Cannabis Act and associated Cannabis Regulations and Industrial Hemp Regulations come into force on October 17, 2018.
Tags: Cannabis
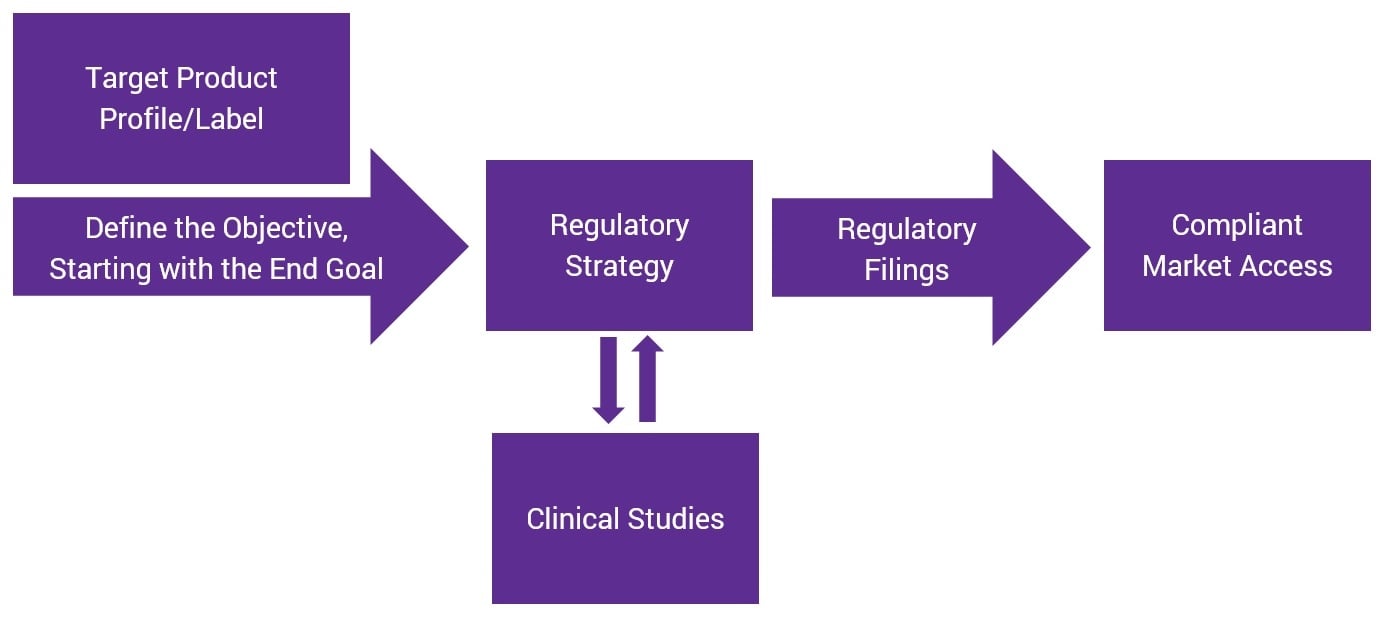
Reverse-Engineering Your Regulatory Strategy for More Efficient Product Development
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Thu, Jul 12, 2018
Regulatory strategy is the backbone of successful product development. Brands that lack a clear regulatory plan at the beginning of the process can face longer times to market and potentially higher costs associated with re-work or failure.
Tags: Clinical Trials, Regulatory, Dietary Supplements/Natural Health Products
How Health Canada’s Upcoming Self-Care Product Regulations Could Impact Your Marketed Natural Health Products, Non-Prescription Drugs, and Cosmetics
Posted by Nutrasource on Wed, Jun 27, 2018
Tags: Product Marketing, Pharmaceuticals, Regulatory, Dietary Supplements/Natural Health Products
Can DNA Testing Solve the Supplement Industry’s Identity Problems?
Posted by David Erickson, Ph.D. on Thu, Jun 21, 2018
It’s surprisingly easy not to notice that you are living in a time of revolution. The technological revolution we are living through has been rumbling along for so long now, sometimes it’s hard to appreciate it or get excited. But the revolution in DNA sequencing and its effects on society are worth our attention – especially when it comes to how we characterize and identify natural products.
Tags: Product Testing & Certifications, Probiotics, Dietary Supplements/Natural Health Products
What to Expect When Conducting Dietary Supplement Clinical Trials
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Tue, Mar 20, 2018
One of the most common questions we hear from clients early in the product lifecycle is: "How do I let my customers know our product is safe, and that it works?"
Tags: Clinical Trials
Nutrasource, GOED, DSM Jointly Publish Largest-Ever Fish Oil Oxidation Study
Posted by Jennifer Andrews, M.Sc., MBA, Marketing Director on Fri, Feb 23, 2018
Fish oil supplements remain popular sources of EPA and DHA omega-3 fatty acids. Some studies have suggested that commercially available fish oil supplements are excessively oxidized, impacting oil quality and safety.
To investigate this issue, Nutrasource's scientific team paired with the Global Organization for EPA and DHA Omega-3s (GOED) and DSM to commission the largest study ever to evaluate oxidation parameters in a large database of fish oil omega-3 dietary supplements, and to compare the data with other common commercially available dietary oils.
Tags: Product Testing & Certifications, Omega-3s, Dietary Supplements/Natural Health Products
Let's Connect at 2018's Most Buzzworthy Natural Health Events
Posted by Jennifer Andrews, M.Sc., MBA, Marketing Director on Fri, Feb 02, 2018
It's official: 2018 is going to be big. Our calendar is jam-packed with the year's hottest events in dietary supplements, foods, omega-3s, and probiotics.
We're always ready to help companies overcome barriers on the pathway to market. It doesn't matter if your product is new or existing, food or pharma, early or late in the product lifecycle, or at any stage of the supply chain - our team is here to help!
Schedule a meeting with us at one of the following events to learn how we can help you launch your product with confidence.
Tags: Events, Probiotics, Dietary Supplements/Natural Health Products
Understanding the Clinical Trials Pathway for Probiotics
Posted by Josh Baisley, B.Sc., Vice President, Clinical Design & Delivery on Thu, Jan 04, 2018
Making the decision to conduct a clinical trial can be daunting. Many complex steps are involved in getting a study off the ground, including designing the study, assessing risk, and determining whether a trial will achieve return on investment.
Tags: Probiotics